- Ways to "treat" the battery
- Desulfation
- Why does battery sulfation occur?
- Reasons for this process
- temperature fluctuations
- Low temperature
- high air temperature
- Critical electrolyte drop
- Dead battery
- deep discharge
- Frequent high current charging
- Desulfation with charger
- Battery desulfation with a special charger
- Reverse charging method
- Sulfation of battery plates - what is it?
- The main causes of sulfation
- How to eliminate plate sulfation
- Chemical additives
- Electrochemical method
- Sulfation of battery plates - how to fix?
- Do-it-yourself battery desulfation
- Do-it-yourself recovery with a simple charger
- Instructions for charging the battery with a conventional charger
- Causes of sulfation of car battery plates
- Sulfation
- Signs of violations in this process
- How to check the battery
Ways to "treat" the battery
After discovering problems with the battery, the driver wonders if he needs to buy a new one or is it possible to restore the old battery.
Let's look at which batteries are repairable and which are not.
You should not waste time on the battery if:
- the battery has obvious mechanical damage;
- the cause of the failure is not related to the sulfation process. It can be, for example, closed banks or plates simply collapsed.
If all of the above signs of sulfation are clearly visible, then you can try to bring the battery back to life.
Desulfation
Desulfation is a process aimed at cleaning the plates from deposits of lead sulfate crystals in various ways.
- With the use of a special charger. This method requires the purchase of a special charger that has a charge-discharge mode of operation. Such devices cost about 5000 rubles. The desulfation process itself is quite simple. We remove the battery from the car and connect it to the device. We leave the battery in this state for a long time - sometimes this process can even take several days. The screen of the charger will display information to what level it was possible to restore the battery capacity. It is somewhat more difficult to understand how things are with “treatment” if the charger does not have a display.

Desulfator for car battery
- mechanical cleaning. Sometimes there are craftsmen who advise you to try to disassemble the battery and manually clean the plates from plaque. This method is suitable only for very experienced craftsmen and will require a lot of time and skills.
- Chemical cleaning. Some motorists advise cleaning the plates with special solutions that can dissolve sulfate. It happens like this:
- all electrolyte present in the battery is drained;
- the cleaning solution is immediately poured in and left for about an hour. The solution may begin to boil and splash out;
- drain the solution and rinse the battery several times with distilled water;
- fill in new electrolyte.
With a good set of circumstances, the battery capacity and its performance will be fully restored.But this method has one rather significant drawback - it is very aggressive. Problems can arise if the plates are too worn. In the process of such cleaning, they can completely collapse. Another danger in this case may be fallen lead particles, which can bridge the plates under the influence of the solution, which will also completely disable the battery.
- With a normal charger. This is the most optimal way of desulfation, which is ideal for not too advanced cases.
we check the electrolyte level and, if necessary, add distilled water to the battery. The solution should completely cover all plates
It is important to remember that in this case neither electrolyte nor concentrate can be added;
we need a charger with indicators "Volt" and "Amp" and connect our battery to it;
set Volts - 14-14.3 and Amps 0.8-1 and leave for about 8-12 hours;
we check the indicators - the density should remain the same, and the voltage should rise to 10 volts;
without fail we leave the battery alone for a day;
again put on charge for 8 hours, but with a current of 2-2.5 Amperes;
Let's check the scores again. The voltage will be at the level of 12.7 volts
Density may rise slightly to 1.13;
Let's start the unloading process. We need a high beam lamp or something similar. We connect it to the battery and leave it for about 8 hours until the voltage drops to 9V. It is very important! The density should remain at the same level;
then we repeat the entire charging algorithm - the density should increase to 1.17.

The process of discharging the charging must be carried out several times, here it is very important to achieve a density of 1.27 g / cm3. This method may require you from 8 to 14 days, but the battery will be restored by about 90%, and there is practically no risk of harm here.
This method may require you from 8 to 14 days, but the battery will be restored by about 90%, and there is practically no risk of harm here.
Why does battery sulfation occur?
If the battery is often used during incomplete charging, then it gradually loses capacity due to such a phenomenon as plate sulfation, but not everyone knows what it is and what it means for the battery. Consider the chemical reactions that take place in the process of sulfation.
During operation, lead sulfate settles on the battery plates. The gradual loss of charge is characterized by the following chemical reaction: Pb + 2H2SO4 + PbO2 → 2PbSO4 + 2H2O. This means that lead plates with lead oxide on the surface come into contact with each other, and sulfuric acid is also involved in this reaction. As a result, lead sulfate is formed, as well as water.
When connected to Vympel 55 or another battery charger, the reaction occurs exactly the opposite, and lead sulfate disappears, and the density of the electrolyte increases. But not always to the end, it may remain on the plates, especially if the battery is far from new. Thus, the useful surface of the battery is contaminated and reduced. Lead sulfate has poor electrical conductivity, and the capacity of a sulfated battery decreases.
Because of what sulfation can occur faster and more often:
- the car is idle for a long time without use;
- the battery is rarely charged from the network, thus reducing the number of back reactions;
- The battery is stored for a long time in a state of complete discharge;
- discharge "to zero" - modern calcium batteries are such that in this case their electrodes are covered with calcium sulfate and stop charging to the end;
- on the contrary, recharging the battery - keeping the battery connected to the network for a long time;
- work in the "city mode" - frequent starts and short periods in motion;
- work in "extreme" conditions - too low or too high (from + 40 ° C) air temperature.
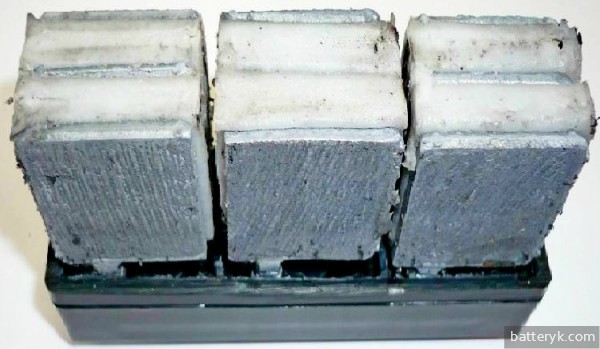
How to determine that the plates are sulfated? First of all, this is noticed when the battery begins to lose capacity. Starting to investigate the reasons for this, you can find a specific white coating on the battery plates, which looks like snow. Other signs are heating of the plates, boiling of the battery when charging ahead of time, too high potential on the electrodes. All of which means it's time for desulfation - unless you want to avoid a complete car battery replacement, of course.
Reasons for this process
The reasons for the deposition of crystals on the plates can be completely different. Most often it is:
- temperature fluctuations;
- critical decrease in electrolyte;
- a long period of being in a discharged state;
- deep discharge;
- frequent charging with high currents.
temperature fluctuations
In this situation, the main role is played not just by low or very high temperatures, but by their strong differences. Everything happens according to the following scheme.
Lead sulfate dissolves in sulfuric acid with great difficulty, for this it is necessary to increase the temperature significantly.During heating, sulfate dissolves in the electrolyte.
After the electrolyte has cooled, sulfate in the form of crystals will fall out again and settle on the plates.
If, during the heating process, the crystals do not dissolve completely, then new ones will first of all settle in these places, which will gradually turn small crystals into rather large ones that cannot be dissolved on their own.
In such a situation, "positive" plates most often suffer and crystals form in deeper porous layers.
Low temperature
In addition to simple temperature fluctuations, low temperatures also affect the condition of the battery plates, albeit in combination with frequent and short trips. Every driver knows that with a large "minus" the car needs more energy to start, and the battery charges much more slowly. With frequent short trips, the car does not have time to warm up well, and the battery does not get enough charge, so sooner or later it will reach a critically low charge. It is this factor that negatively affects the sulfation process.
high air temperature
High ambient temperatures can also adversely affect the condition of the plates. The battery under such conditions operates at a temperature of about 60 degrees and all chemical processes in it occur as quickly as possible. Thus, the sulfation process that has already begun will proceed at an accelerated pace.
Critical electrolyte drop
According to the regulations, the battery plates must always be completely covered with electrolyte.After some time of intensive use of the car, the electrolyte level may drop and the plates may be partially exposed. If the owner of the car does not notice this in time, then after a while in these open areas the process of formation of sulfate crystals will begin, which will gradually become very strong and cannot be destroyed.

Dead battery
Sometimes, out of inexperience, drivers believe that if the battery is not used, then there will be no deposits on the plates, alas, this is not at all the case. When the battery is stored for a long time in a discharged state, it gradually loses part of its capacity, which provokes the formation of crystalline deposits on the plates. But the reverse process of dissolving these crystals does not occur. Thus, sulfation problems are almost inevitable, and it will be very difficult to correct the situation.
deep discharge
All discharges of the battery can be brought to acceptable levels, which is approximately 1.75-1.80 V
It is important to remember here that the lower the discharge current, the greater the final voltage can be achieved.
The battery pack consists of several batteries, and they wear out a little differently, their capacity starts to vary. If the battery is put on a full charge for batteries with a larger capacity, then the weaker ones will receive an excess charge, that is, a deep discharge. When discharged, they will not be able to completely get rid of crystalline deposits, and these formations will grow with each excessive discharge.
It is important to remember that with a deep discharge, sulfation occurs almost instantly and after 1-2 such discharges to save the battery, you have to take urgent measures.
Frequent high current charging
If you often use a large current when charging the battery, then you may encounter a situation where the lead sulfate on the plates does not have time to completely dissolve. This will continue from charge to charge and gradually the battery capacity will become too small for further use.
Desulfation with charger
Unlike chemical, do-it-yourself electrochemical methods of battery desulfation do not require either disassembling the battery or draining the electrolyte. To get rid of sulfation, it is enough to use the usual charger, which is available in the household of most car owners.

An example of a common algorithm for proper battery desulfation using a conventional charger:
we discharge the battery until the density of the electrolyte decreases to a value of 1.04–1.07 g / cm³;
set the current to the memory at 0.8–1.1 A, the voltage should be in the range of 13.9–14.3 V;
we charge the battery with such parameters for about 8 hours;
let the battery "rest" throughout the day;
charge the battery again for 8 hours, increasing the current to 2.0–2.6 A at the same voltage level;
we discharge the battery again using a powerful external load for 8 hours - the voltage at the terminals should drop to a minimum of 9 volts (make sure that it is not less, this is important);
repeat steps 2–5 as many times as necessary until the density of the electrolyte reaches the nominal value of 1.27 g/cm³.
This method can take from several days to several weeks, but it is considered the most optimal, with an efficiency of about 80-90%.
Battery desulfation with a special charger
On sale there are also special chargers with a built-in desulfation mode.As a rule, these are automatic chargers that you just need to connect to the battery and select the appropriate function. No additional action is needed, but in this case the procedure will be lengthy. Depending on the degree of sulfation of the plates, it can last 3-7 days, during which you will not be able to use the battery.
Reverse charging method
Removing lead sulfate plaque using this method is a very risky procedure, so it can only be recommended in cases where other methods have proven ineffective.

We'll need DC source high power, for example, an old-style welding machine with output voltage characteristics up to 20 V at a current strength of 80 A.
The battery removed from the car with the plugs unscrewed is connected to the power supply in the reverse way (minus to plus and vice versa). We turn on the source to the network and charge the battery for about 30 minutes. The electrolyte will boil intensively, but since it must be replaced, we do not pay attention to it.
It remains to drain the remaining electrolyte, fill in a new solution and charge the battery with a conventional charger.
Sulfation of battery plates - what is it?

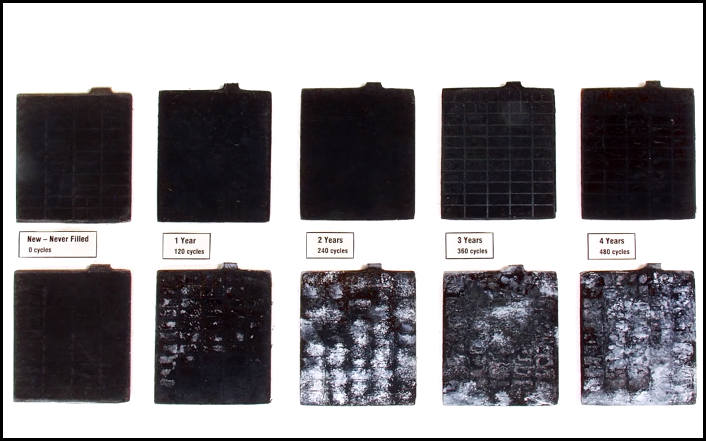
When the battery is discharged, a natural process of sulfation of the active mass of the battery plates occurs. In this case, lead sulfate of a fine crystalline structure is formed, which dissolves when the battery is charged.
But if the battery mode is as described below, then a different kind of sulfation occurs. The resulting large crystals of lead sulfate isolate the active mass.
The more these crystals formed, the less the working surface of the active mass, and hence the battery capacity. Outwardly, they can be seen as a white coating on lead plates.
What are the dangers for the normal functioning of the battery? Let's figure it out right away. Do you drive and have there been any problems with the battery?
About the causes of battery sulfation, video.
The main causes of sulfation
- At least in the fall and spring, remove the battery, charge it and monitor the electrolyte density for the season, if not, this is the first reason.
- Drive every day, the car does not stand in the parking lot for half a month, and the engine, from the moment it was started until the moment it was turned off, runs at medium speed for at least half an hour, if not, this is the second reason.
- And you don’t get into traffic jams, and the engine doesn’t overheat, if not, this is the third reason.
- When you stop the car, always turn off the light, if not, this is the fourth reason.
These are the main reasons that can lead to such a sad phenomenon as battery sulfation.
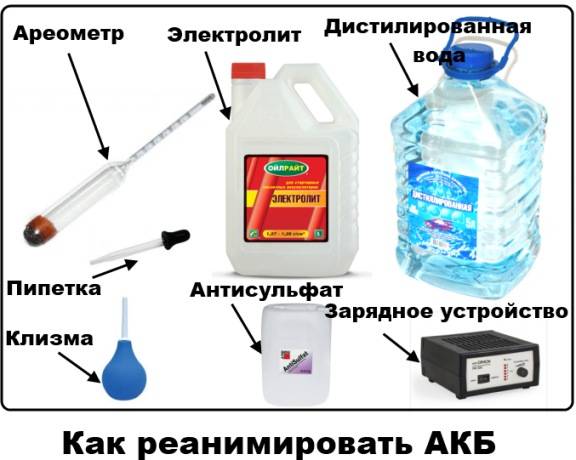
If the battery is sulphated, there is no need to immediately go to choose a new one. Try to restore it. This procedure takes quite a lot of time, but not complicated, as it seems at first glance. This will require a hydrometer, a charger and a measuring device that allows you to measure voltage and current.
How to eliminate plate sulfation
Desulfation is understood as the effect on electrodes and plates in various ways that help to eliminate the formed plaque of calcium or lead salts.There are such types of cleaning: mechanical, chemical or with the use of inorganic additives, electrochemical with the use of a charger.

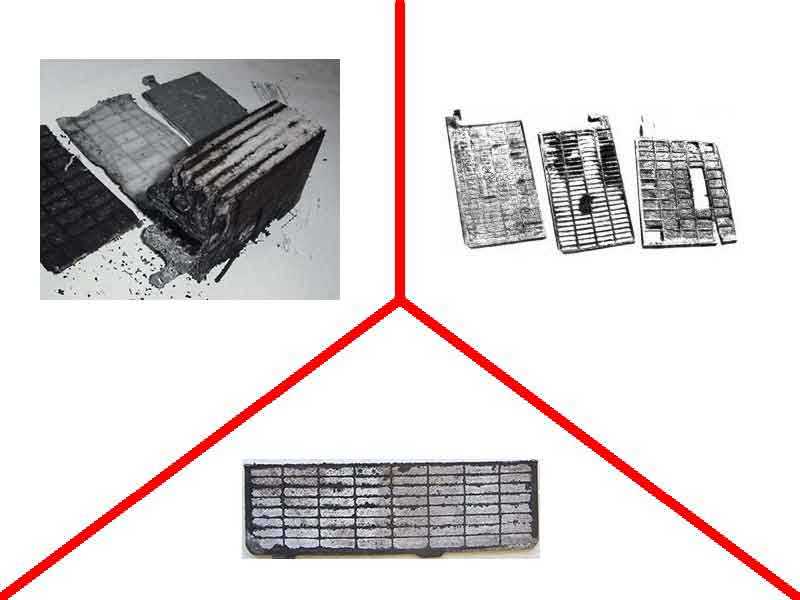
The simplest and fastest way of desulfation is the mechanical cleaning of the plates from the formed salt crystals. Batteries of the old type or serviced allow you to remove the cover and gain access to the plates and electrodes.
These components are removed from the battery manually and cleaned in the same way - the plaque is simply scraped off the surface and cracks until it is completely eliminated as far as possible. Modern units are more often produced in an unattended sample. This makes it impossible to get to the banks with electrodes in order to get them and clean them.
To clean the plates of a dead battery by this method, it is necessary to perform a number of operations:
Remove or cut off the upper part of the case for serviced batteries
Clean each of the plates manually, carefully so as not to damage the structure of the electrodes;
Install the cleaned plates in their place in the containers, observing the required gap between each;
Make the case airtight, solder the removed cover;
Fill the jars with electrolyte of the required density;
Conduct a battery performance test, "adjust" the density of the liquid to the same level in all banks, avoiding a spacing of more than 0.01 kg / cu. cm and the electrolyte concentration is not lower than 1.25, but not higher than 1.31 kg / cu.
cm.
For EFB batteries, this method is not applicable, since each group of electrodes is separately soldered in a separator designed to prevent flaking of the plates.

In this design, the density of the electrolyte in the bank and the package itself (separator) differ, which will ruin the device after breaking the integrity. This factor prevents mechanical desulfation.
Chemical additives
The essence of the process is the introduction of special additives with a chemical composition that acts on calcium or lead sulfates into the cavity of the electrolyte jars. During charging, solutions with additives slow down the formation of salt deposits on the electrodes, which returns the battery to an almost nominal charge.
Most often, Trilon-B is chosen, but this solution does not work equally effectively on all batteries. The reaction depends on the design features of the battery, model and technical parameters. There is a 50/50 chance that chemical desulfation will work.
The composition of Trilon-B includes 5% ammonia, 2% acid of an organic derivative of sodium salt, distillate. These components are inert to lead, but react well with plaque on the electrodes. In industry, such a solution is used to convert insoluble salts into soluble ones.

Procedure for chemical desulfation:
- In accordance with the above proportions, the Trilon-B solution is prepared
- The battery is fully charged
- 2-3 times the battery cans are flushed with distillate
- The solution must spend at least an hour in the cavity of the cans so that chemical reactions end and gases stop being released
- The inactive solution is drained upon completion of the reactions (pumped out without turning the device over)
- Rinse the inside of the jars 1-2 times with distilled water
- New electrolyte, density 1.25-1.27 kg/cu.cm, is poured into each jar, its density is checked and adjusted to one value with a spacing of not more than 0.01 kg / cu. cm for each container
- The battery is fully charged, the liquid concentration is adjusted
Electrochemical method
The most productive method of desulfation is electrochemical, which is carried out by a special charger.
The essence of electrical desulfation is to pass a current through the electrolyte with higher rates than the nominal values of the battery. This leads to natural dissolution in the liquid surrounding the plates of accumulations of lead or calcium salts and dissolution in it, increasing the density of the electrolyte. This brings the battery performance back to normal.
Sulfation of battery plates - how to fix?

So, the main problem with lead-acid batteries with sulfuric acid electrolyte is sulfation. While the plaque is insignificant, it can be removed at home. The crystals clogged the porous surface of the lead. You can extract them only by decomposing them into ions and directing them to different electrodes. Used:
- exposure to reverse currents or battery recovery with pulsed charges;
- desulfation with a small current for a long time;
- chemical sludge solvents;
- mechanical descaling of the plates.
At home, to eliminate battery sulfation, you can use a long exposure to the battery with a current of 2-3 A, preventing the cans from boiling. The procedure is carried out for 24 hours and beyond until the electrolyte density is stable for 5-6 hours. Conducting 2-3 training cycles can return the capacity to 80% of an incompletely clogged battery.
The ferrous sulfate precipitate dissolves well in a solution of ethylenediaminetetraacetic acid (trilon B). The lead in the salt is replaced by a sodium ion and it becomes soluble. The solution is prepared in the ratio of 60 g of Trilon B powder + 662 ml of NH4OH 25% + 2340 ml distilled water.
To remove sulfation, pour the solution into the battery for 60 minutes, immediately after removing the electrolyte. The reaction in the jars is violent, with heating and boiling. Then drain the solution, rinse the cavities 3 times with distilled water and fill in fresh electrolyte. If the lead plates do not break down, a complete cleaning of the plates will occur.
Light plaque can be removed using distilled water. The contents of the cans must be completely removed by draining into an enamel bowl. If there are coal chips in the contents of the jar, it will not recover, the plates are destroyed.
Fill the jars with electrolyte, leave the plugs open, connect the charger, set the voltage to 14 V. Ensure that the boiling in the jars is moderate and leave for a week or two under load. The dissolved precipitate turns water into a weak electrolyte. To get rid of sulfation, repeat the procedure several times. Finish cleaning as soon as all the sediment on the battery plates has dissolved.
Single and double polarity reversal is used in cases where other cleaning methods have not helped. Changing the charge of the plates will help dissolve the precipitate by changing the direction of electron movement. But this method will destroy the battery with thin lead plates. It does not apply to modern budget models made in China.
When using special additives that dissolve the sediment, it is necessary to follow the instructions exactly, carry out work in a ventilated room, use personal protective equipment.

Do-it-yourself battery desulfation
An equally effective way to remove lead sulphate is to wash the cans with chemically active substances. As you know, acidic compounds react with alkali, therefore, to carry out do-it-yourself desulfation using chemistry, you will need to purchase a suitable reagent.
With the task of splitting sulfate plaque, baking soda will help to cope. For the procedure it is necessary:
- Drain the electrolyte from the battery.
- Dissolve lye in distilled water in a ratio of 1 to 3.
- Heat the mixture to a boil.
- Pour the hot alkaline solution into the battery jars for 30-40 minutes.
- Drain the alkaline solution.
- Rinse the battery at least 3 times with clean hot water.
- Pour electrolyte into jars.
If the procedure for chemical desulfation of the plates was carried out carefully, then the battery capacity will increase significantly. It can be used for a long time, until plaque forms again on the plates.

Do-it-yourself recovery with a simple charger
You can desulfate the battery yourself using a special or standard charger.
A conventional charger can be automatic with the ability to regulate the currents and voltages supplied to the terminals and the “Desulfation” mode or simplified with the need to control the process. The most convenient option is an automatic pulse charger with a desulfation mode.
Charging steps with an automatic charger with desulfation mode include the following steps:
- The negative and positive terminals of the automatic device are connected to the corresponding poles of the battery;
- The required voltage and the strength of the supplied current are adjusted, the “Desulfation” mode is turned on;
- Equipment is connected to the network;
- The battery begins to charge, the process of resuming the plates occurs on the negative terminal;
- At the end of the charging process until its capacity and electrolyte density are fully restored, the power supply is disconnected, the battery terminals of the automatic device are removed.
Process time depends on many factors:
- The degree of discharge of the battery;
- Equipment capacities;
- The level of electrode sulfation.
To calculate the average charge time, divide the battery capacity by the average charge current. Most often, it takes from 15 hours to 3 days to fully restore the equipment.
Instructions for charging the battery with a conventional charger
This type of electrochemical battery charging requires regular monitoring of the process and continuous intervention. For the reliability and accuracy of charging, the instruction is designed for a battery with an electrolyte density of 1.07 g / cu. cm and a voltage of 8 V at the terminals of the equipment. Without receiving voltage, this appliance begins to boil after 15 minutes with a typical charge.
For desulfation, do the following:
- Provide a room with good air circulation for charging the device;
- Check the electrolyte level in the battery banks and replenish it, if necessary, with distilled water;
- Connect the battery to the charger;
- Set the current with a power of 0.8-1 A and a voltage of 13.9-14.3 V for about 8-9 hours.These manipulations will raise the voltage at the battery terminals to 10 V, leaving the electrolyte density level unchanged;
- Disconnect the battery from the charger and keep it in this state for about a day;
- The battery is reconnected to the charger with new current parameters: a power of 2-2.5 A and a voltage of 13.9-14.3 V for 8-9 hours;
- After recharging, the battery parameters will change: the density of the electrolyte will increase to 1.12 g / cu. cm, and the voltage at the terminals will rise to 12.8 V;
- This indicates the beginning of desulfation. For the next step, you need to discharge the battery to the 9 V mark by connecting to the active resistance terminals - a lamp or a headlight. The average time for discharge is 8-9 hours. The density of the electrolytic liquid will be kept at 1.12 g/cu. cm;
It is necessary to control the process of discharging the battery, since the final voltage must remain at least 9 V.
A subsequent pair of charging and discharging the battery according to the above scenario will increase the electrolyte level to a value of 1.16 g / cu. cm. It is necessary to repeat the cycle until the density reaches 1.26 g / cu. cm or does not come close to the nominal 1.27 g / cu. cm.
As practice shows, such manipulations update the battery by 80-90%.
Causes of sulfation of car battery plates
As mentioned above, the main cause of sulfation is a deep discharge of the battery, but by no means the only one. Let's consider in detail all the available reasons:
Deep discharge of the battery.If we analyze the above-described process of “crystals” sticking to the battery plates, we can conclude that when the battery is deeply discharged, sulfation occurs without fail. A full charge of the battery will correct the situation, but even with it, the battery will lose a little in capacity.
It is important to know that having allowed the battery to completely discharge 1-3 times, you can immediately look for a replacement for it, since it will not be able to gain more than the required capacity;
Low temperatures and short trips. Motorists are well aware that in frosty weather, you must first take care of the safety of the battery.
As such, low temperature does not affect the sulfation process of the plates, but it affects indirectly. In the cold season, starting the engine by spinning the starter requires more energy than at a positive ambient temperature. In addition, in the cold, the battery during the trip is worse charged. This problem is especially relevant when it comes to short trips. In fact, when starting the engine, the driver spends a large amount of energy, after which, after 15-20 minutes, he turns off the engine, and the car does not have enough time to warm up and charge the battery;
Heat. Not only low ambient temperature negatively affects the battery, but also high. In the hot season, the battery has to work at temperatures above 60 degrees Celsius. Due to such high temperatures, all chemical processes in it proceed faster, including sulfation. Therefore, in the hot season, it is recommended to keep the battery as charged as possible so that plaque does not form on the plates;
Use of concentrated electrolyte or sulfuric acid.Some drivers try to remove plaque accumulated on the plates with concentrated sulfuric acid or electrolyte. Under no circumstances should this be done. Thus, it will not be possible to “melt” the formed “crystals”, but only the process of their formation will be aggravated;
Storage of a discharged battery. Another oversight that inexperienced drivers sin. As you know, the chemical processes in the battery do not stop, even when it is disconnected from the consumer. Accordingly, if you store a battery discharged for several months, it will lose some capacity during this time. As we found out above, with a loss of capacity, lead sulfate adheres to the plates, that is, the process of sulfation. And since there is no battery charge, the “crystals” will not “melt”, and there is a high risk of critical sulfation, in which it will no longer be possible to restore the battery capacity.
As can be seen from the above, most of the causes are simply sulfation catalysts. In fact, it occurs in the battery all the time, but only with critical sulfation does the situation become almost irreversible for the battery.
Sulfation
Sulfation is the process of deposits of lead and calcium salts on the plates of a car battery. This reaction occurs throughout the use of the power supply, but with proper operation it does not cause negative consequences. Only under certain conditions does a process become malicious.
At the moment of filling the electrolyte into the battery, the production of very small crystals of lead sulfate immediately begins, which settle on the plates and form a thin film.If the power supply is working properly, then with further recharging of the battery, this film will again be converted into an electrolyte.
In case of violations in the operation of the battery, the crystals on the plates become larger and gradually cover the entire working surface of the plates, practically clogging them. In this situation, the reverse process of the transition of crystals into the electrolyte does not occur. Such a process will very soon clearly affect the operation of the car.
Signs of violations in this process
The first signs that drivers pay attention to are:
- gradual decrease in battery capacity;
- fast charging and discharging of the unit;
- battery banks can boil quite quickly;
- electrolyte indicators are very low;
- even with a fully charged battery, it is almost impossible to start the car, and a simple headlight bulb puts the battery to “zero” in just a few minutes;
- the driver has a feeling of insufficient current, that is, there is a decrease in the brightness of the headlights, poor air conditioning, etc.
Sometimes the driver can observe only a few signs of incorrect operation of the power supply, and sometimes they appear all at once.
How to check the battery
The process of sulfation of the battery plates can be seen simply by examining them.
It is important to understand here that the inspection should only be carried out with a fully charged battery. This is due to the fact that uncharged plates always show signs of sulfation.
- in a battery that is in good condition, the plates are clean and silver. They are easily distinguishable from black separators;
- in the case of a process that has already begun, the “negative” plates acquire a white-gray tint, but the “positive” plates at the same time become brownish with clear white spots. If already at this stage no action is taken to “treat” the battery, then the process will go further and the minus plates will begin to bulge clearly, and the plus ones will warp. This is due to uneven mechanical stress. As a result of such changes, a very large loss of battery capacity occurs.