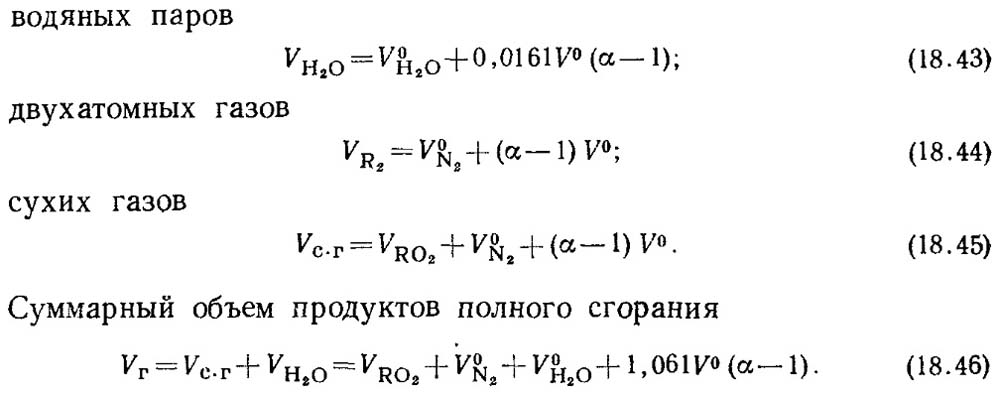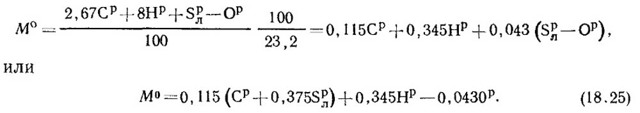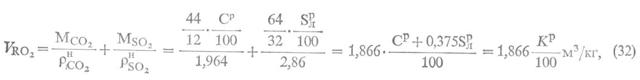- 2.2 Sulfur oxides
- Appendix E. Examples of calculation of emissions of harmful substances from the combustion of associated petroleum gas
- General principles for calculating heating power and energy consumption
- And why are such calculations carried out at all?
- How to find out the gas consumption for heating a house
- How to reduce gas consumption
- How to calculate main gas consumption
- Calculation for liquefied gas
- Consumption of liquefied propane-butane mixture
- The formula for calculating the consumption of a combustible mixture
- An example of calculating the consumption of liquefied gas
- How to calculate gas consumption for home heating
- Method of calculation for natural gas
- Appendix G. Torch Length Calculation
- Method of calculation for natural gas
- We calculate the gas consumption by heat loss
- Heat loss calculation example
- Boiler power calculation
- By quadrature
- Appendix C. Calculation of the stoichiometric combustion reaction of associated petroleum gas in an atmosphere of humid air (section 6.3).
- Appendix E1. Calculation examples
- Annex A. Calculation of the physical and chemical characteristics of associated petroleum gas (clause 6.1)
- Appendix B. Calculation of the physicochemical characteristics of moist air for given weather conditions (clause 6.2)
- Gas consumption for DHW
- Conclusions and useful video on the topic
2.2 Sulfur oxides
The total amount of sulfur oxides MSO2emitted into the atmosphere with flue gases (g/s, t/year),
calculated according to the formula
where B is the consumption of natural fuel for the period under consideration,
g/s (t/year);
Sr - sulfur content in the fuel for the working mass,%;
η'SO2 - share
sulfur oxides bound by fly ash in the boiler;
η"SO2_share of sulfur oxides,
collected in the wet ash collector along with the capture of solid particles.
guide values η'SO2when burning various types of fuel are:
Fuel η'SO2
peat……………………………………………………………………………….. 0.15
Estonian and Leningrad shales…………………………………. 0.8
slates of other deposits…………………………………………… 0.5
Ekibastuz coal………………………………………………………….. 0.02
Berezovsky coals of Kansk-Achinsk
basin
for furnaces with solid slag removal……………….. 0.5
for furnaces with liquid slag removal………………… 0.2
other coals of Kansk-Achinsk
basin
for furnaces with solid slag removal……………….. 0.2
for furnaces with liquid slag removal……………….. 0.05
coals from other deposits……………………………………………….. 0.1
fuel oil……………………………………………………………………………… 0.02
gas……………………………………………………………………………………. 0
The share of sulfur oxides (η"SO2) captured in dry ash collectors is taken equal to
zero. In wet ash collectors, this proportion depends on the total alkalinity of the irrigation water.
and from the reduced sulfur content of the fuel Spr.
(36)
At the specific water consumption for operation, typical for
irrigation of ash collectors 0.1 – 0.15 dm3/nm3η"SO2determined by the drawing of the Appendix.
In the presence of hydrogen sulfide in the fuel, the value of the sulfur content on
working mass Sr in the formula
() value is added
∆Sr=0.94
H2S, (37)
where H2S is the content of hydrogen sulfide in the fuel per working mass,%.
Note. —
When developing standards for maximum permissible and temporarily agreed
Emissions (MPE, VSV), it is recommended to apply the balance-calculation method, which allows
account more accurately for sulfur dioxide emissions. This is due to the fact that sulfur
distributed unevenly in the fuel. When determining the maximum emissions in
grams per second, the maximum Sr values are used
actually used fuel. At
in determining gross emissions in tons per year, average annual values are used
Sr.
Appendix E. Examples of calculation of emissions of harmful substances from the combustion of associated petroleum gas
1. Associated petroleum gas of the Yuzhno-Surgutskoye field. Gas volume flow Wv = 432000 m3 / day = 5 m3 / s. Soot-free combustion, gas density () rG = 0.863 kg/m3. Mass flow is ():
Wg = 3600rGWv = 15534 (kg/h).
In accordance with and emissions of harmful substances in g / s are:
CO, 86.2 g/s; NOx — 12.96 g/s;
benzo(a)pyrene - 0.1 10-6 g / s.
to calculate hydrocarbon emissions in terms of methane, their mass fraction is determined based on and . It is equal to 120%. The underburn is 6 104. That. methane emission is
0.01 6 10-4 120 15534 = 11.2 g/s
Sulfur is absent in APG.
2. Associated petroleum gas of the Buguruslan field with the conditional molecular formula C1.489H4.943S0.011O0.016. Gas volume flow Wv = 432000 m/day = 5 m/s. The flare device does not provide soot-free combustion. Gas density () rG = 1.062 kg/m3. Mass flow is ():
Wg = 3600 rGWv = 19116 (kg/h).
In accordance, and emissions of harmful substances in g / s are:
CO - 1328 g/s; NOx — 10.62 g/s;
benzo(a)pyrene - 0.3 10-6 g/s.
Sulfur dioxide emissions are determined by , where s = 0.011, mG = 23.455 mSO2 = 64. Hence
MSO2 = 0.278 0.03 19116 = 159.5 g/s
In this case, underburning is 0.035. Mass content of hydrogen sulfide 1.6%. From here
MH2S = 0.278 0.035 0.01 1.6 19116 = 2.975 g/s
Hydrocarbon emissions are determined similarly to example 1.
General principles for calculating heating power and energy consumption
And why are such calculations carried out at all?
The use of gas as an energy carrier for the functioning of the heating system is advantageous from all sides. First of all, they are attracted by quite affordable tariffs for "blue fuel" - they cannot be compared with the seemingly more convenient and safe electric one. In terms of cost, only affordable types of solid fuels can compete, for example, if there are no special problems with harvesting or acquiring firewood. But in terms of operating costs - the need for regular delivery, organization of proper storage and constant monitoring of the boiler load, solid fuel heating equipment completely loses to gas connected to the mains supply.
In a word, if it is possible to choose this particular method of heating a home, then it is hardly worth doubting the expediency of installing a gas boiler.
According to the criteria of efficiency and ease of use, gas heating equipment currently has no real rivals
It is clear that when choosing a boiler, one of the key criteria is always its thermal power, that is, the ability to generate a certain amount of thermal energy.To put it simply, the purchased equipment, according to its inherent technical parameters, should ensure the maintenance of comfortable living conditions in any, even the most unfavorable conditions. This indicator is most often indicated in kilowatts, and, of course, is reflected in the cost of the boiler, its dimensions, and gas consumption. This means that the task when choosing is to purchase a model that fully meets the needs, but, at the same time, does not have unreasonably high characteristics - this is both unprofitable for the owners and not very useful for the equipment itself.
When choosing any heating equipment, it is very important to find a "golden mean" - so that there is enough power, but at the same time - without its completely unjustified overestimation
It is important to understand one more thing correctly. This is that the indicated nameplate power of a gas boiler always shows its maximum energy potential.
With the right approach, it should, of course, somewhat exceed the calculated data on the required heat input for a particular house. Thus, the very operational reserve is laid down, which, perhaps, will someday be needed under the most unfavorable conditions, for example, during extreme cold, unusual for the area of \u200b\u200bresidence. For example, if calculations show that for a country house the need for thermal energy is, say, 9.2 kW, then it would be wiser to opt for a model with a thermal power of 11.6 kW.
Will this capacity be fully demanded? - it is quite possible that it is not. But its stock does not look excessive.
Why is this explained in such detail? But only to ensure that the reader has clarity with one important point. It would be completely wrong to calculate the gas consumption of a particular heating system, based solely on the passport characteristics of the equipment. Yes, as a rule, in the technical documentation accompanying the heating unit, the energy consumption per unit of time (m³ / h) is indicated, but again this is more of a theoretical value. And if you try to get the desired consumption forecast by simply multiplying this passport parameter by the number of hours (and then days, weeks, months) of operation, then you can come to such indicators that it will become scary!..
It is not advisable to take passport values of gas consumption as a basis for calculations, since they will not show the real picture
Often, the consumption range is indicated in the passports - the boundaries of the minimum and maximum consumption are indicated. But this, probably, will not be of great help in carrying out calculations of real needs.
But it is still very useful to know the gas consumption as close to reality as possible. This will help, firstly, in planning the family budget. And secondly, the possession of such information should, voluntarily or involuntarily, encourage zealous owners to search for energy saving reserves - perhaps it is worth taking certain steps to reduce consumption to the possible minimum.
How to find out the gas consumption for heating a house
How to determine the gas consumption for heating a house 100 m 2, 150 m 2, 200 m 2?
When designing a heating system, you need to know what it will cost during operation.
That is, to determine the upcoming fuel costs for heating. Otherwise, this type of heating may subsequently be unprofitable.
How to reduce gas consumption
A well-known rule: the better the house is insulated, the less fuel is spent on heating the street. Therefore, before starting the installation of the heating system, it is necessary to perform high-quality thermal insulation of the house - the roof / attic, floors, walls, replacing windows, hermetic sealing contour on the doors.
You can also save fuel by using the heating system itself. Using warm floors instead of radiators, you will get more efficient heating: since heat is distributed by convection currents from the bottom up, the lower the heater is located, the better.
In addition, the normative temperature of floors is 50 degrees, and radiators - an average of 90. Obviously, floors are more economical.
Finally, you can save gas by adjusting the heating over time. It makes no sense to actively heat the house when it is empty. It is enough to withstand a low positive temperature so that the pipes do not freeze.
Modern boiler automation (types of automation for gas heating boilers) allows remote control: you can give a command to change the mode through a mobile provider before returning home (what are Gsm modules for heating boilers). At night, the comfortable temperature is slightly lower than during the day, and so on.
How to calculate main gas consumption
The calculation of gas consumption for heating a private house depends on the power of the equipment (which determines the gas consumption in gas heating boilers). Power calculation is performed when choosing a boiler.Based on the size of the heated area. It is calculated for each room separately, focusing on the lowest average annual temperature outside.
To determine the energy consumption, the resulting figure is divided approximately in half: throughout the season, the temperature fluctuates from a serious minus to plus, gas consumption varies in the same proportions.
When calculating the power, they proceed from the ratio of kilowatts per ten squares of the heated area. Based on the foregoing, we take half of this value - 50 watts per meter per hour. At 100 meters - 5 kilowatts.
Fuel is calculated according to the formula A = Q / q * B, where:
- A - the desired amount of gas, cubic meters per hour;
- Q is the power required for heating (in our case, 5 kilowatts);
- q - minimum specific heat (depending on the brand of gas) in kilowatts. For G20 - 34.02 MJ per cube = 9.45 kilowatts;
- B - the efficiency of our boiler. Let's say 95%. The required figure is 0.95.
We substitute the numbers in the formula, we get 0.557 cubic meters per hour for 100 m 2. Accordingly, gas consumption for heating a house of 150 m 2 (7.5 kilowatts) will be 0.836 cubic meters, gas consumption for heating a house of 200 m 2 (10 kilowatts) - 1.114, etc. It remains to multiply the resulting figure by 24 - you get the average daily consumption, then by 30 - the average monthly.
Calculation for liquefied gas
The above formula is also suitable for other types of fuel. Including for liquefied gas in cylinders for a gas boiler. Its calorific value, of course, is different. We accept this figure as 46 MJ per kilogram, i.e. 12.8 kilowatts per kilogram. Let's say the boiler efficiency is 92%. We substitute the numbers in the formula, we get 0.42 kilograms per hour.
Liquefied gas is calculated in kilograms, which are then converted to liters.To calculate the gas consumption for heating a house of 100 m 2 from a gas tank, the figure obtained by the formula is divided by 0.54 (the weight of one liter of gas).
Further - as above: multiply by 24 and by 30 days. To calculate the fuel for the entire season, we multiply the average monthly figure by the number of months.
Average monthly consumption, approximately:
- consumption of liquefied gas for heating a house of 100 m 2 - about 561 liters;
- consumption of liquefied gas for heating a house of 150 m 2 - approximately 841.5;
- 200 squares - 1122 liters;
- 250 - 1402.5 etc.
A standard cylinder contains about 42 liters. We divide the amount of gas required for the season by 42, we find the number of cylinders. Then we multiply by the price of the cylinder, we get the amount needed for heating for the entire season.
Consumption of liquefied propane-butane mixture
Not all owners of country houses have the opportunity to connect to a centralized gas pipeline. Then they get out of the situation using liquefied gas. It is stored in gas tanks installed in the pits, and replenished using the services of certified fuel supply companies.
Liquefied gas used for domestic purposes is stored in sealed containers and reservoirs - propane-butane cylinders with a volume of 50 liters, or gas tanks
If liquefied gas is used to heat a country house, the same calculation formula is taken as the basis. The only thing - it must be borne in mind that bottled gas is a mixture of brand G30. In addition, the fuel is in the state of aggregation. Therefore, its consumption is calculated in liters or kilograms.
The formula for calculating the consumption of a combustible mixture
A simple calculation will help to estimate the cost of a liquefied propane-butane mixture.The initial data of the building are the same: a cottage with an area of 100 squares, and the efficiency of the installed boiler is 95%.

When calculating, it should be taken into account that fifty-liter propane-butane cylinders, for the purpose of safety, fill no more than 85%, which is about 42.5 liters
When performing the calculation, they are guided by two significant physical characteristics of the liquefied mixture:
- bottled gas density is 0.524 kg/l;
- the heat released during the combustion of one kilogram of such a mixture is equal to 45.2 MJ / kg.
To facilitate calculations, the values \u200b\u200bof the released heat, measured in kilograms, are converted into another unit of measurement - liters: 45.2 x 0.524 \u003d 23.68 MJ / l.
After that, the joules are converted to kilowatts: 23.68 / 3.6 \u003d 6.58 kW / l. To obtain correct calculations, the same 50% of the recommended power of the unit is taken as a basis, which is 5 kW.
The obtained values \u200b\u200bare substituted into the formula: V \u003d 5 / (6.58 x 0.95). It turns out that the consumption of the G 30 fuel mixture is 0.8 l / h.
An example of calculating the consumption of liquefied gas
Knowing that in one hour of operation of the boiler generator, an average of 0.8 liters of fuel is consumed, it will not be difficult to calculate that one standard cylinder with a 42-liter filling volume will last approximately 52 hours. This is a little more than two days.
For the entire heating period, the consumption of the combustible mixture will be:
- For a day 0.8 x 24 \u003d 19.2 liters;
- For a month 19.2 x 30 = 576 liters;
- For a heating season lasting 7 months 576 x 7 = 4032 liters.
For heating a cottage with an area of 100 squares, you will need: 576 / 42.5 \u003d 13 or 14 cylinders. For the entire seven-month heating season, 4032/42.5 = from 95 to 100 cylinders will be needed.

To accurately calculate the number of propane-butane cylinders needed to heat the cottage during the month, you need to divide the monthly volume of 576 liters consumed by the capacity of one such cylinder
A large volume of fuel, taking into account transportation costs and creating conditions for its storage, will not be cheap. But still, in comparison with the same electric heating, such a solution to the issue will still be more economical, and therefore preferable.
How to calculate gas consumption for home heating
Gas is still the cheapest type of fuel, but the cost of connection is sometimes very high, so many people want to first assess how economically justified such costs are. To do this, you need to know the gas consumption for heating, then it will be possible to estimate the total cost and compare it with other types of fuel.
Method of calculation for natural gas
The approximate gas consumption for heating is calculated based on half the capacity of the installed boiler. The thing is that when determining the power of a gas boiler, the lowest temperature is laid. This is understandable - even when it is very cold outside, the house should be warm.
You can calculate the gas consumption for heating yourself
But it is completely wrong to calculate the gas consumption for heating according to this maximum figure - after all, in general, the temperature is much higher, which means that much less fuel is burned. Therefore, it is customary to consider the average fuel consumption for heating - about 50% of the heat loss or boiler power.
We calculate the gas consumption by heat loss
If there is no boiler yet, and you estimate the cost of heating in different ways, you can calculate from the total heat loss of the building. They are most likely familiar to you. The methodology here is as follows: they take 50% of the total heat loss, add 10% to provide hot water supply and 10% to heat outflow during ventilation. As a result, we get the average consumption in kilowatts per hour.
Then you can find out the fuel consumption per day (multiply by 24 hours), per month (by 30 days), if desired - for the entire heating season (multiply by the number of months during which the heating works). All these figures can be converted into cubic meters (knowing the specific heat of combustion of gas), and then multiply cubic meters by the price of gas and, thus, find out the cost of heating.
Heat loss calculation example
Let the heat loss of the house be 16 kW / h. Let's start counting:
- average heat demand per hour - 8 kW / h + 1.6 kW / h + 1.6 kW / h = 11.2 kW / h;
- per day - 11.2 kW * 24 hours = 268.8 kW;
- per month - 268.8 kW * 30 days = 8064 kW.
The actual gas consumption for heating still depends on the type of burner - modulated are the most economical
Convert to cubic meters. If we use natural gas, we divide the gas consumption for heating per hour: 11.2 kW / h / 9.3 kW = 1.2 m3 / h. In calculations, the figure 9.3 kW is the specific heat capacity of natural gas combustion (available in the table).
By the way, you can also calculate the required amount of fuel of any type - you just need to take the heat capacity for the required fuel.
Since the boiler has not 100% efficiency, but 88-92%, you will have to make more adjustments for this - add about 10% of the figure obtained. In total, we get the gas consumption for heating per hour - 1.32 cubic meters per hour. You can then calculate:
- consumption per day: 1.32 m3 * 24 hours = 28.8 m3/day
- demand per month: 28.8 m3 / day * 30 days = 864 m3 / month.
The average consumption for the heating season depends on its duration - we multiply it by the number of months that the heating season lasts.
This calculation is approximate. In some month, gas consumption will be much less, in the coldest - more, but on average the figure will be about the same.
Boiler power calculation
Calculations will be a little easier if there is a calculated boiler capacity - all the necessary reserves (for hot water supply and ventilation) are already taken into account. Therefore, we simply take 50% of the calculated capacity and then calculate the consumption per day, month, per season.
For example, the design capacity of the boiler is 24 kW. To calculate the gas consumption for heating, we take half: 12 k / W. This will be the average need for heat per hour. To determine the fuel consumption per hour, we divide by the calorific value, we get 12 kW / h / 9.3 k / W = 1.3 m3. Further, everything is considered as in the example above:
- per day: 12 kW / h * 24 hours = 288 kW in terms of the amount of gas - 1.3 m3 * 24 = 31.2 m3
- per month: 288 kW * 30 days = 8640 m3, consumption in cubic meters 31.2 m3 * 30 = 936 m3.
You can calculate gas consumption for heating a house according to the design capacity of the boiler
Next, we add 10% for the imperfection of the boiler, we get that for this case the flow rate will be slightly more than 1000 cubic meters per month (1029.3 cubic meters). As you can see, in this case everything is even simpler - fewer numbers, but the principle is the same.
By quadrature
Even more approximate calculations can be obtained by the quadrature of the house. There are two ways:
Appendix G. Torch Length Calculation
Torch length (Lf) is calculated by the formula:
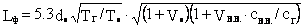 ,(1)
,(1)
where dabout is the diameter of the mouth of the flare unit, m;
TG - combustion temperature, ° K ()
Tabout — — temperature of combusted APG, °K;
VV.V. — the theoretical amount of moist air required for the complete combustion of 1m3 APG (), m3/m3;
rV.V.rG - the density of moist air () and APG ();
Vo — stoichiometric amount of dry air for burning 1 m3 of APG, m3/m3:
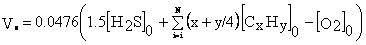
where [H2S]about, [CxHy]o, [O2]o - the content of hydrogen sulfide, hydrocarbons, oxygen, respectively, in the combusted hydrocarbon mixture, % vol.
On - shows nomograms for determining the length of the torch (Lf) related to the diameter of the mouth of the flare unit (d), depending on ТG/Tabout, VBB and rBBrG for four fixed values TG/Tabout with variation ranges VBB 8 to 16 and rBB/RG from 0.5 to 1.0.
Method of calculation for natural gas
The approximate gas consumption for heating is calculated based on half the capacity of the installed boiler. The thing is that when determining the power of a gas boiler, the lowest temperature is laid. This is understandable - even when it is very cold outside, the house should be warm.
You can calculate the gas consumption for heating yourself
But it is completely wrong to calculate the gas consumption for heating according to this maximum figure - after all, in general, the temperature is much higher, which means that much less fuel is burned. Therefore, it is customary to consider the average fuel consumption for heating - about 50% of the heat loss or boiler power.
We calculate the gas consumption by heat loss
If there is no boiler yet, and you estimate the cost of heating in different ways, you can calculate from the total heat loss of the building. They are most likely familiar to you. The methodology here is as follows: they take 50% of the total heat loss, add 10% to provide hot water supply and 10% to heat outflow during ventilation.As a result, we get the average consumption in kilowatts per hour.
Then you can find out the fuel consumption per day (multiply by 24 hours), per month (by 30 days), if desired - for the entire heating season (multiply by the number of months during which the heating works). All these figures can be converted into cubic meters (knowing the specific heat of combustion of gas), and then multiply cubic meters by the price of gas and, thus, find out the cost of heating.
| The name of the crowd | unit of measurement | Specific heat of combustion in kcal | Specific heating value in kW | Specific calorific value in MJ |
|---|---|---|---|---|
| Natural gas | 1 m 3 | 8000 kcal | 9.2 kW | 33.5 MJ |
| Liquefied gas | 1 kg | 10800 kcal | 12.5 kW | 45.2 MJ |
| Hard coal (W=10%) | 1 kg | 6450 kcal | 7.5 kW | 27 MJ |
| wood pellet | 1 kg | 4100 kcal | 4.7 kW | 17.17 MJ |
| Dried wood (W=20%) | 1 kg | 3400 kcal | 3.9 kW | 14.24 MJ |
Heat loss calculation example
Let the heat loss of the house be 16 kW / h. Let's start counting:
- average heat demand per hour - 8 kW / h + 1.6 kW / h + 1.6 kW / h = 11.2 kW / h;
- per day - 11.2 kW * 24 hours = 268.8 kW;
-
per month - 268.8 kW * 30 days = 8064 kW.
Convert to cubic meters. If we use natural gas, we divide the gas consumption for heating per hour: 11.2 kW / h / 9.3 kW = 1.2 m3 / h. In calculations, the figure 9.3 kW is the specific heat capacity of natural gas combustion (available in the table).
Since the boiler has not 100% efficiency, but 88-92%, you will have to make more adjustments for this - add about 10% of the figure obtained. In total, we get the gas consumption for heating per hour - 1.32 cubic meters per hour. You can then calculate:
- consumption per day: 1.32 m3 * 24 hours = 28.8 m3/day
- demand per month: 28.8 m3 / day * 30 days = 864 m3 / month.
The average consumption for the heating season depends on its duration - we multiply it by the number of months that the heating season lasts.
This calculation is approximate. In some month, gas consumption will be much less, in the coldest - more, but on average the figure will be about the same.
Boiler power calculation
Calculations will be a little easier if there is a calculated boiler capacity - all the necessary reserves (for hot water supply and ventilation) are already taken into account. Therefore, we simply take 50% of the calculated capacity and then calculate the consumption per day, month, per season.
For example, the design capacity of the boiler is 24 kW. To calculate the gas consumption for heating, we take half: 12 k / W. This will be the average need for heat per hour. To determine the fuel consumption per hour, we divide by the calorific value, we get 12 kW / h / 9.3 k / W = 1.3 m3. Further, everything is considered as in the example above:
- per day: 12 kW / h * 24 hours = 288 kW in terms of the amount of gas - 1.3 m3 * 24 = 31.2 m3
-
per month: 288 kW * 30 days = 8640 m3, consumption in cubic meters 31.2 m3 * 30 = 936 m3.
Next, we add 10% for the imperfection of the boiler, we get that for this case the flow rate will be slightly more than 1000 cubic meters per month (1029.3 cubic meters). As you can see, in this case everything is even simpler - fewer numbers, but the principle is the same.
By quadrature
Even more approximate calculations can be obtained by the quadrature of the house. There are two ways:
- It can be calculated according to SNiP standards - for heating one square meter in Central Russia, an average of 80 W / m2 is required. This figure can be applied if your house is built according to all requirements and has good insulation.
- You can estimate according to the average data:
- with good house insulation, 2.5-3 cubic meters / m2 are required;
-
with average insulation, gas consumption is 4-5 cubic meters / m2.
Each owner can assess the degree of insulation of his house, respectively, you can estimate what gas consumption will be in this case. For example, for a house of 100 sq. m. with average insulation, 400-500 cubic meters of gas will be required for heating, 600-750 cubic meters per month for a house of 150 square meters, 800-100 cubic meters of blue fuel for heating a house of 200 m2. All this is very approximate, but the figures are based on many factual data.
Appendix C. Calculation of the stoichiometric combustion reaction of associated petroleum gas in an atmosphere of humid air (section 6.3).
1. The stoichiometric combustion reaction is written as:
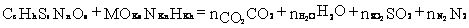 (1)
(1)
2. Calculation of the molar stoichiometric coefficient M according to the condition of complete saturation of the valency (completely completed oxidation reaction):
where vj‘ and vj- valency of elements j and j', which are part of moist air and APG;
kj‘ and kj - the number of atoms of elements in the conditional molecular formulas of moist air and gas ( and ).
3. Determination of the theoretical amount of moist air VB.B. (m3/m3) required for complete combustion of 1 m3 of APG.
In the equation of the stoichiometric combustion reaction, the molar stoichiometric coefficient M is also the coefficient of the volumetric ratios between the fuel (associated petroleum gas) and the oxidizer (moist air); complete combustion of 1 m3 of APG requires M m3 of humid air.
4. Calculation of the amount of combustion products VPS (m3/m3) formed during the stoichiometric combustion of 1 m3 of APG in an atmosphere of humid air:
VPS=c + s + 0.5[h + n + M(kh + kn)],(3)
where c, s, h, n and kh, kn correspond to the conditional molecular formulas of APG and moist air, respectively.
Appendix E1. Calculation examples
Calculation of specific CO emissions2, H2O, N2 and O2 per unit mass of flared associated petroleum gas (kg/kg)
Associated petroleum gas of the Yuzhno-Surgutskoye field with the conditional molecular formula C1.207H4.378N0.0219O0.027 () is burned in an atmosphere of humid air with the conditional molecular formula O0.431N1.572H0.028 () for a = 1.0.
Molar stoichiometric coefficient M=11.03 ().
Specific emission of carbon dioxide ():
Specific water vapor emission H2O:
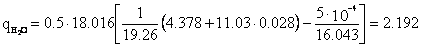
Specific nitrogen emission N2:
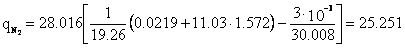
Specific oxygen emission O2:
Example 2
Associated petroleum gas of the Buguruslan field with the conditional molecular formula C1.489H4.943S0.011O0.016.
Gas combustion conditions are the same as in. Specific emission of carbon dioxide ().
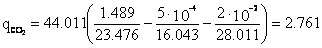
Specific water vapor emission H2O:
Specific nitrogen emission N2:

Specific oxygen emission O2:
Annex A. Calculation of the physical and chemical characteristics of associated petroleum gas (clause 6.1)
1. Calculation of density rG (kg/m3) APG by volume fractions Vi (% vol.) () and density ri (kg/m3) () components:
2. Calculation of the conditional molecular weight of APG mG, kg/mol ():
where mi is the molecular weight of the i-th component of APG ().
3. Calculation of the mass content of chemical elements in associated gas ():
The mass content of the j-th chemical element in APG bj (% wt.) is calculated by the formula:
,(3)
where bij is the content (% wt.) of the chemical element j in the i-th component of APG ();
bi is the mass fraction of the ith component in APG; 6i calculated by the formula:
bi=0.01VirirG(4)
Note: if hydrocarbon emissions are determined in terms of methane, the mass fraction of hydrocarbons converted to methane is also calculated:
b(SWithH4)i=SbimimcH4
In this case, the summation is carried out only for hydrocarbons that do not contain sulfur.
4. Calculation of the number of atoms of elements in the conditional molecular formula of associated gas ():
The number of atoms of the jth element Kj calculated by the formula:
The conditional molecular formula of associated petroleum gas is written as:
CCHhSSNnOO(6)
where c=Kc, h=Kh, s=Ks, n= Kn, o=Ko, are calculated by formula (5).
Appendix B. Calculation of the physicochemical characteristics of moist air for given weather conditions (clause 6.2)
1. Conditional molecular formula for dry air
O0.421N1.586,(1)
what does the conditional molecular weight correspond to
mS.V.=28.96 kg/mol
and density
rS.V.=1.293 kg/m3.
2. Mass moisture content of humid air d (kg/kg) for a given relative humidity j and temperature t, °C at normal atmospheric pressure is determined by ().
3. Mass fractions of components in moist air ():
- dry air; (2)
- moisture (H2O)(3)
4. Content (% wt.) of chemical elements in the components of moist air
Table 1.
| Component | The content of chemical elements (% mass) | ||
| O | N | H | |
| Dry air O0.421N1.586 | 23.27 | 76.73 | — |
| Moisture H2O | 88.81 | — | 11.19 |
5. Mass content (% wt.) of chemical elements in moist air with moisture content d
Table 2.
| Component | G | Dry air O0.421N1.586 | Moisture H2O | S |
| O | 23.27 1+d | 88.81d 1+d | 23.27 + 88.81d 1+d | |
| bi | N | 76.73 1+d | — | 76.73 1+d |
| H | — | 11.19d 1+d | 11.19d 1+d |
6. The number of atoms of chemical elements in the conditional molecular formula of moist air ()
| Element | O | N | H |
| ToJ | 0.421 + 1.607d 1+d | 1.586 1+d | 3.215d 1+d |
Conditional molecular formula of moist air:
OCo.nKn·NKh(4)
5. Density of humid air depending on weather conditions. At a given temperature of moist air t, °C, barometric pressure P, mm Hg. and relative humidity j, the density of humid air is calculated by the formula:
where RPis the partial pressure of water vapor in air, depending on t and j; is determined.
Gas consumption for DHW
When water for household needs is heated using gas heat generators - a column or a boiler with an indirect heating boiler, then to find out the fuel consumption, you need to understand how much water is required. To do this, you can raise the data prescribed in the documentation and determining the rate for 1 person.
Another option is to turn to practical experience, and it says the following: for a family of 4 people, under normal conditions, it is enough to heat 80 liters of water once a day from 10 to 75 ° C. From here, the amount of heat required for heating water is calculated according to the school formula:
Q = cmΔt, where:
- c is the heat capacity of water, is 4.187 kJ/kg °С;
- m is the mass flow rate of water, kg;
- Δt is the difference between the initial and final temperatures, in the example it is 65 °C.
For the calculation, it is proposed not to convert volumetric water consumption into mass water consumption, assuming that these values are the same. Then the amount of heat will be:
4.187 x 80 x 65 = 21772.4 kJ or 6 kW.
It remains to substitute this value in the first formula, which will take into account the efficiency of the gas column or heat generator (here - 96%):
V \u003d 6 / (9.2 x 96 / 100) \u003d 6 / 8.832 \u003d 0.68 m³ of natural gas 1 time per day will be spent on heating water. For a complete picture, here you can also add the consumption of a gas stove for cooking at the rate of 9 m³ of fuel per 1 living person per month.
Conclusions and useful video on the topic
The video material attached below will allow you to identify the lack of air during gas combustion without any calculations, that is, visually.
It is possible to calculate the amount of air required for efficient combustion of any volume of gas in a matter of minutes.And owners of real estate equipped with gas equipment should keep this in mind. Since at a critical moment when the boiler or any other appliance will not work properly, the ability to calculate the amount of air needed for efficient combustion will help identify and fix the problem. What, moreover, will increase security.
Would you like to supplement the above material with useful information and recommendations? Or do you have any billing questions? Ask them in the comment block, write your comments, take part in the discussion.