- Causes and danger of gas leakage
- Explosiveness of natural gas
- Mining methods
- Composition of natural gas
- Carbon dioxide and hydrogen sulfide
- inert gases
- Origin
- Main properties of odorants
- Natural gas production
- GB poison gas
- Natural gas production:
- Methods of treatment and prevention
- Prevention
- Gas odorization
- Natural gas:
- Natural gas odorization methods
- Method #1 - Drip Substance Injection
- Method #2 - Using a Wick Odorizer
- Method # 3 - bubbling odor injection into gas
- Safety measures when working with mercaptans
- The process of adding odor to gas
Causes and danger of gas leakage
Careless attitude when installing gas equipment can lead to gas leakage in the apartment. At the same time, two types of leak causes are distinguished: domestic accidents and professional flaws.
With a professional defect, there may be:
- defects in pipes and gas pipelines;
- flaws in gas columns;
- balloon damage;
- broken burner;
- poor or incorrect fastening of the hose and the appearance of creases and cracks;
- violation of tightness in fastening the thread of the nut that connects the plate to the hose;
- wear or other defects in the hose gasket or seal material on the faucet.
 Flaws in geysers can cause gas leaks
Flaws in geysers can cause gas leaks
In the case of such leaks, it is impossible to immediately determine why it smells like gas. In domestic conditions, other reasons are also possible, which are most often associated with the human factor:
- the tap is not closed or poorly closed;
- the fire on the stove or in the oven has gone out, but the gas continues to flow.
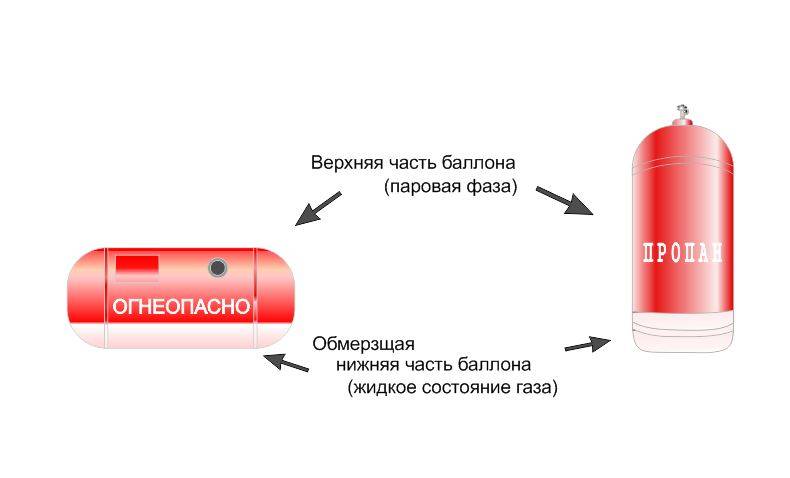
The main danger of natural gas is that it has a neutral odor and is colorless. However, in order to timely detect a leak, manufacturers add special additives to the gas that have a specific pungent odor.
The personal feelings of a person who has been poisoned by household gas include: headache, nausea, suffocation, dizziness, dry mouth, tearing, burning and redness of the eyes, general weakness, impaired appetite and sleep, etc. With a large accumulation of gas in a closed room with access to oxygen and other explosive sources (fire, electricity, etc.), an explosion and collapse of the room will most likely occur.
Explosiveness of natural gas

What kind of gas in the apartment is explosive or not? The concentration of fuel for the occurrence of the effect of its ignition is an extremely fine value. The probability of an explosion depends on the composition of the gas, the pressure level, and the ambient temperature.
A dangerous situation can occur only if the concentration of natural fuel in the room reaches 15% in relation to the total air mass.
It is impossible to independently determine the percentage of gas in space without the use of specialized measuring equipment. Therefore, having felt a characteristic aroma, it is necessary to shut off the fuel supply to household appliances
It is also extremely important to de-energize devices that use electrical impulses. This applies not only to household appliances, but also to devices that operate on batteries, batteries.
As practice shows, when the gas concentration in the room is at the level of 15% of the total amount of air, its ignition can occur even from the operation of a mobile phone or laptop.
If you smell gas, you must immediately open all doors and windows in the room. Ventilation of housing will reduce the likelihood of an explosion before the arrival of an emergency service.
Mining methods
Extraction of natural gas is carried out according to certain techniques and methods. The thing is that the depth of its occurrence can reach several kilometers. In such conditions, a specially designed program and new, modern and powerful equipment are required.
The production technique is based on creating a pressure difference in the gas reservoir and the outside atmospheric air. As a result, with the help of a well, the product is pumped out of the places of occurrence, and the reservoir is saturated with water.
Wells are drilled along a certain trajectory resembling a ladder. This is done because:
- this saves space and preserves the integrity of materials during production, since gas impurities (hydrogen sulfide, for example) are very harmful to the equipment;
- this allows you to distribute the pressure on the formation more evenly;
- in this way it is possible to penetrate to a depth of up to 12 km, which makes it possible to study the lithospheric composition of the earth's interior.
As a result, natural gas production becomes quite successful, uncomplicated and well organized. Once the product has been retrieved, it is shipped to its destination.If this is a chemical plant, then there it is cleaned and prepared for further use in various industries.
In particular, for household purposes, it is necessary not only to clean the product, but also to add odorants to it - special substances that give a sharp unpleasant odor. This is done for safety reasons in case of leaks in the premises.

Composition of natural gas

Natural gases are mainly represented by methane - CH4 (up to 90 - 95%). It is the simplest gas in terms of chemical formula, combustible, colorless, lighter than air. The composition of natural gas also includes ethane, propane, butane and their homologues. Combustible gases are an obligatory companion of oils, forming gas caps or dissolving in oils.
- Methane
- Carbon dioxide and hydrogen sulfide
- Nitrogen
- inert gases
Carbon dioxide and hydrogen sulfide
Carbon dioxide and hydrogen sulfide in the gas mixture appear mainly due to the oxidation of hydrocarbons under surface conditions with the help of oxygen and with the participation of aerobic bacteria.
At great depths, when hydrocarbons come into contact with natural sulfate formation waters, both carbon dioxide and hydrogen sulfide are formed.
For its part, hydrogen sulfide easily enters into oxidative reactions, especially under the influence of sulfur bacteria, and then pure sulfur is released.
Thus, hydrogen sulfide, sulfur and carbon dioxide constantly accompany hydrocarbon gases.
CO2 in gases ranges from fractions to several percent, but deposits of natural gas with a carbon dioxide content of up to 80 - 90% are known.
hydrogen sulfide in gases is also from fractions of a percent to 1 - 2%, but there are gases with a high content of it. Examples are the Orenburg field (up to 5%), Karachaganakskoye (up to 7-10%), Astrakhanskoye (up to 25%).At the same Astrakhan field, the share of carbon dioxide reaches 20%.
inert gases
Inert gases - helium, argon and others, like nitrogen, do not react and are found in hydrocarbon gases, as a rule, in small quantities.
The background values of the helium content are 0.01 - 0.15%, but there are also up to 0.2 - 10%. An example of the industrial content of helium in natural hydrocarbon gas is the Orenburg field. To extract it, a helium plant was built next to the gas processing plant.
Origin
There are two theories of the origin of natural gas: mineral and biogenic.
According to the mineral theory, hydrocarbons are formed as a result of a chemical reaction deep in the bowels of our planet from inorganic compounds under the influence of high pressures and temperatures. Further, due to the internal dynamics of the Earth, hydrocarbons rise to the zone of least pressure, forming deposits of minerals, including gas.
According to the biogenic theory, natural gas was formed in the bowels of the Earth as a result of anaerobic decomposition of organic substances of plant and animal origin under the influence of high temperatures and pressures.
Despite the ongoing debate regarding the origin of hydrocarbons, the biogenic theory wins in the scientific community.
Main properties of odorants
Gas is widely used in everyday life and can provoke severe poisoning, and its high concentration creates an explosive environment.Initially, household gas (methane with other impurities, including propane, ethane, butane) is odorless, and any leak from a closed system could only be detected using special sensors.
This problem is solved by adding a component with a pronounced odor to the gas - an odorant. And the direct process of entering the stream is called odorization. Mixing is carried out at the gas distribution station or at centralized points.
Ideally, odorants should have the following properties:
- Have a pronounced, specific smell for a clear and quick recognition.
- Ensure stable dosage. When mixed with methane and moving through a gas pipe, odorants must exhibit chemical and physical resistance.
- Have a sufficient level of concentration to reduce the total consumption.
- Do not form toxic products during operation.
- Additives should not exhibit a corrosive effect in relation to tanks, fittings, which will ensure a long service life of gas equipment and pipelines.
There is no odorant that fully meets all these criteria. Therefore, technical specifications TU 51-31323949-94-2002 and Regulations for the operation of VRD 39-1.10-069-2002 were developed for Gazprom. But these are Gazprom's internal documents that are mandatory for execution only by organizations that are part of the Gazprom Group.
The document VRD 39-1.10-06-2002 contains the basic requirements for the manufacture, storage, transportation and use of additives.
 To neutralize the strong smell of the odorant in the places of its leakage, a solution of potassium permanganate or bleach is used. In this case, you will definitely need a gas mask and other protective equipment.
To neutralize the strong smell of the odorant in the places of its leakage, a solution of potassium permanganate or bleach is used. In this case, you will definitely need a gas mask and other protective equipment.
The correct use of odorants is regulated in the Rules for the operation of main gas pipelines STO Gazprom 2-3.5-454-2010, which states that the explosive limit of a flammable liquid is 2.8-18%, and the MPC is 1 mg / m3.
 To determine the intensity of the odor of the odorant in points, as well as to measure its mass concentration, the gas analyzer ANKAT-7631 Micro-RSH can be used.
To determine the intensity of the odor of the odorant in points, as well as to measure its mass concentration, the gas analyzer ANKAT-7631 Micro-RSH can be used.
Inhalation of vapors can cause vomiting, loss of creation, in large quantities the substance causes convulsions, paralysis and death. According to the degree of impact on the body, these are harmful substances of the 2nd hazard class. To determine their concentration in the room, you can use the gas analyzer type RSH.
Natural gas production
Methods for the production of gaseous hydrocarbons are similar to oil production - gas is extracted from the bowels using wells. In order for the formation pressure of the deposit to drop gradually, wells are placed evenly throughout the entire territory of the deposit. This method also prevents the occurrence of gas flows between areas of the field and premature flooding of the deposit.
More details in the article: Extraction of natural gas.
According to a BP report, in 2017, global natural gas production amounted to 3,680 bcm. The United States became the leader in production - 734.5 billion m3, or 20% of the total world figure. Russia took the second place with 635.6 bcm.
GB poison gas
This substance is better known as sarin. In September 2013, the UN confirmed that a chemical weapons attack using specially designed rockets that spread sarin gas at rebels in a suburb of the Syrian capital took place a month earlier.UN Secretary-General Ban Ki-moon says this is the most significant confirmed use of chemical weapons against civilians since Saddam Hussein used it in Halabja in 1988.
Sarin gas is a volatile but toxic phosphorus-based nerve agent. One drop the size of a pinhead is enough to quickly kill an adult human. This colorless, odorless liquid retains its state of aggregation at room temperature, but quickly evaporates when heated. Once released, it rapidly spreads into the environment. As with VX, symptoms include headache, salivation and tearing followed by gradual muscle paralysis and possible death.
Sarin was developed in 1938 in Germany when scientists were researching pesticides. The Aum Shinrikyo cult used it in 1995 on the Tokyo subway. Although the attack caused widespread panic, it only killed 13 people because the agent was sprayed in liquid form. To maximize wastage, sarin must not only be a gas, but the particles must be small enough to be easily absorbed through the lining of the lungs, but heavy enough that they are not exhaled.

Natural gas production:
Natural gas deposits are located deep in the earth, at a depth of one to several kilometers. Therefore, in order to extract it, it is necessary to drill a well. The deepest well has a depth of more than 6 kilometers.
In the bowels of the Earth, gas is found in microscopic voids - the pores that some rocks possess. The pores are interconnected by microscopic channels - cracks.In pores and cracks, the gas is under high pressure, which is much higher than atmospheric pressure. Natural gas moves in pores and cracks, flowing from high pressure pores to lower pressure pores.
When drilling a well, gas, due to the action of physical laws, completely enters the well, tending to the low pressure zone. Thus, the pressure difference in the field and on the Earth's surface is a natural driving force that pushes gas out of the depths.
Gas is extracted from the bowels of the earth with the help of not one, but several or more wells. Wells are trying to be placed evenly throughout the field for a uniform drop in reservoir pressure in the deposit. Otherwise, gas flows between areas of the deposit are possible, as well as premature flooding of the deposit.
Since the extracted gas contains a lot of impurities, it is cleaned immediately after production using special equipment, after which it is transported to the consumer.
Methods of treatment and prevention
Treatment must be carried out in a hospital. First of all, the victim is connected to an oxygen cylinder for several hours. Then they carry out the necessary examinations and select the appropriate drugs.
Medicines:
- Anti-inflammatory drugs will not allow the spread of inflammation in the respiratory tract;
- Anticonvulsants will help get rid of spasmodic manifestations in the muscles;
- If necessary, use painkillers;
- Be sure to use a complex of vitamins;
- Sorbents contribute to the rapid removal of toxins from the body.
Treatment is carried out until the full restoration of the functioning of the organs.The development of negative consequences is possible, however, with proper and timely treatment, the prognosis is favorable.
Prevention
It is possible to avoid poisoning with any gas if safety precautions are observed. If an unpleasant and foreign smell is felt in the air, it is recommended to leave the room and call the appropriate services. It is forbidden to use a light switch and light a fire in places with an unpleasant smell to avoid a sharp fire.
In the event of gas poisoning, the victim is provided with access to clean air and first aid is provided. A visit to a medical facility is a must.
Gas odorization
Vapors of natural and liquefied hydrocarbon gases are colorless and odorless. This makes it difficult to detect gas in rooms in the event of a leak. According to the requirements of the state standard, the smell of gas should be felt when its volume fraction in the air is 0.5%. To give the gases a specific smell, strongly smelling substances are added to them - odorants, for example, technical ethyl or methyl mercaptan. The average annual consumption rate of mercaptans for natural gas odorization is 16 g (19.1 cm3) per 1000 m3 of gas (at a temperature of 0 °C and a pressure of 760 Pa).
Mercaptans are volatile, colorless liquids with a pronounced specific odor. They can be detected when the content in the air is equal to 2 • 10 9 mg/l. In negligible concentrations, mercaptan vapors cause nausea and headache, and at higher concentrations, they affect the nervous system. In case of mild poisoning with mercaptans, fresh air, rest, strong tea or coffee are recommended; in case of severe nausea, medical assistance is required; in case of respiratory arrest, artificial respiration is required.
As personal protective equipment against mercaptans, a filtering industrial gas mask of grade A is used, and when working in a room with a high concentration of them, insulating hose gas masks with forced air supply, protective sealed goggles, etc.
All equipment when working with odorants must be carefully sealed. Premises in which odorants are stored or used must be equipped with ventilation.
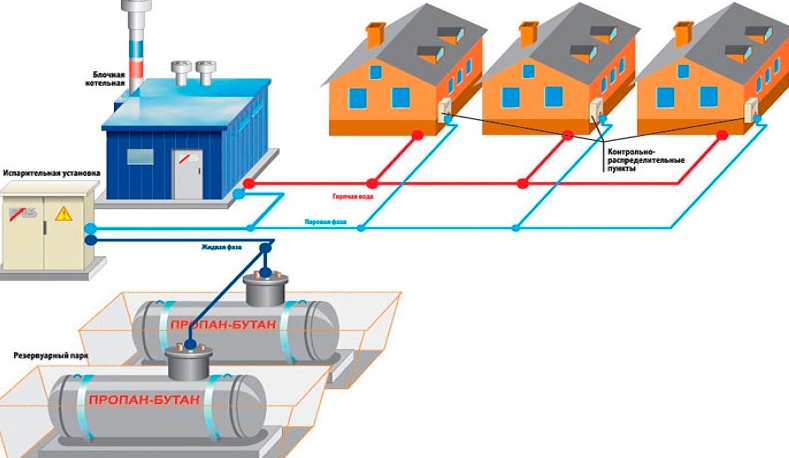
Natural gas odorization are produced at gas distribution stations, liquefied hydrocarbon gases for domestic and domestic purposes - at gas processing, oil refineries and petrochemical plants. With a mass fraction of propane in liquefied gas up to 60% (inclusive), butane and other gases more than 40%, the odorization rate is 60 g of ethylmercaptan per 1 ton of liquefied gas; propane over 60%, butane and other gases up to 40% - 90 g per 1 ton of liquefied gas.
Manufacturers produce odorization in the gas flow by introducing an odorant into pipelines through which gas is pumped from tanks to loading railway racks. Periodically, as well as when complaints are received, the odor intensity of odorized gases is checked by organoleptic and physico-technical methods. At enterprises consuming natural and liquefied hydrocarbon gases for domestic purposes, the intensity of the odor of the odorant in the gas is checked at least once a quarter.
An organoleptic test of the intensity of the odor of odorized gases is carried out by five testers with an assessment on a five-point scale: 0 - no smell; 1—the smell is very weak, indefinite; 2 - the smell is weak, but definite; 3 - moderate smell; 4 - the smell is strong; 5 - the smell is very strong, intolerable.An organoleptic test of the odor intensity of odorized gases is carried out in a specially equipped room-chamber at a temperature of (20 ± 4) ° C, in which the volume fraction of gases in the air should be 0.4%, which corresponds to /b of the lower explosive limit. Gas is admitted into the chamber and mixed with air by means of fans. The smell is considered sufficient if at least three testers give an intensity rating of at least 3 points. If the odor is insufficient, evaluate another gas sample by five disinterested assessors.
At the same time, a physicochemical analysis is performed for the content of ethyl mercaptan in a hydrocarbon gas mixture by one of the following methods: chromatographic, nephelometric, conductometric, bromine index, iodometric.
If domestic gases have their own specific smell, the odorization rate can be reduced.
Odorization plants are classified as explosive, and odorant storage rooms are classified as fire hazardous. During the operation and repair of odorization installations, it is prohibited to carry out work that can cause sparking. It is strictly forbidden to smoke in the room where the odorizing unit is located.
Natural gas:
Natural gas is a mineral, a mixture of gases formed in the bowels of the Earth during the anaerobic decomposition of organic matter.
Natural gas exists in a gaseous, solid or dissolved state.In the first case, in the gaseous state, it is widely distributed and is found in rock layers in the bowels of the Earth in the form of gas deposits (separate accumulations trapped in a "trap" between sedimentary rocks), as well as in oil fields in the form of gas caps. In a dissolved state, it is found in oil and water. In the solid state, it occurs in the form of gas hydrates (the so-called "combustible ice") - crystalline compounds of natural gas and water of variable composition. Gas hydrates are a promising fuel source.
Under normal conditions (1 atm. and 0 °C), natural gas is only in the gaseous state.
It is the cleanest type of fossil fuel. But in order to use it as a fuel, its components are isolated from it for separate use.
Natural gas is a flammable mixture of various hydrocarbons and impurities.
Natural gas is a gaseous mixture consisting of methane and heavier hydrocarbons, nitrogen, carbon dioxide, water vapor, sulfur-containing compounds, inert gases.
It is called natural because it is not synthetic. Gas is born underground in the thickness of sedimentary rocks from the decomposition products of organic matter.
Natural gas is much more widespread in nature than oil.
Has no color or smell. Lighter than air by 1.8 times. Flammable and explosive. When leaking, it does not collect in the lowlands, but rises up.
The characteristic smell of gas used in everyday life is due to odorization - the addition of odorants, that is, unpleasantly smelling substances, to its composition.The most common odorant is ethanethiol, which can be felt in the air at a concentration of 1 per 50,000,000 parts of air. It is thanks to odorization that gas leaks can be easily identified.
Natural gas odorization methods
The type of odorant is chosen based on several requirements:
- required level of accuracy;
- sufficient performance;
- material possibilities.
The additive is used in both liquid and vapor form. The first method involves drip administration or the use of a dosing pump. To saturate with vapors, an odorant is introduced into a part of the gas flow by branching or blowing the wetted wick.
Method #1 - Drip Substance Injection
This input method is characterized by relatively low costs and a simple usage pattern. The principle of operation is based on counting the number of drops per unit of time, which makes it possible to obtain the required flow rate.
To transport gas in large volumes, drops are transformed into a jet of liquid; in such cases, a level gauge scale or a special container with divisions is used.
 The dropper is used for visual control of the consumption of aggressive substances, including when dosing an odorant. All parts, including the body, are made of sustainable materials
The dropper is used for visual control of the consumption of aggressive substances, including when dosing an odorant. All parts, including the body, are made of sustainable materials
This method requires constant manual adjustment and checking of the flow rate, in particular when the number of consumers changes.
The process cannot be automated, so its accuracy is low - it is only 10-25%. In modern installations, the dropper is used only as a reserve in case of a malfunction of the main equipment.
Method #2 - Using a Wick Odorizer
Using a wick odorizer is another method that is suitable for small volumes of gas.All operations are carried out manually. The odorant is used for vapors and liquid state, its content is determined by the amount of consumption per unit of time.
Evaporation in wick odorizers, unlike other devices, occurs directly from the surface over which the gas passes. Coating often consists of flannel wicks
The supply is regulated by changing the amount of gas that is passed through the wick.
Method # 3 - bubbling odor injection into gas
Installations that use bubbling, unlike the previous two, can be automated.
The odorant is supplied using a diaphragm and a dispenser, its amount is calculated in proportion to the gas flow. The substance flows by gravity from the supply tank. The ejector is responsible for the refueling process.
Diagram of a bubbling odorizer. The main elements include a diaphragm, a gas pipeline, a valve, a chamber and a filter. They produce various sizes of devices depending on the performance of the gas distribution station
Among the latest developments to improve the odorization process is the use of dosing pumps. They consist of a cleaning filter, an electronic control unit and a control device - a magnet or a valve.
Safety measures when working with mercaptans
 Odorants designed to prevent emergency situations are themselves explosive and combustible substances of the 2nd hazard class.
Odorants designed to prevent emergency situations are themselves explosive and combustible substances of the 2nd hazard class.
When handling them, the following safety precautions must be observed:
- All manipulations with solutions and equipment in sealed rubberized clothing and a gas mask.
- Double treatment of the soil with neutralizing solutions in case of contact with mercaptans.
- Availability of effective supply and exhaust ventilation in rooms where odorants are stored or used.
- Restriction of access to the room where reagents are stored by unauthorized persons. Reliable locks, locks, security and access control.
- Transportation of liquid by special vehicles equipped with warning signs.
- The presence at the gas distribution station of sensors for detecting gas leaks and odorants, as well as effective fire extinguishing agents.
If liquid is spilled on the floor, it should be immediately fixed with sand, and then transferred to rubber bags for later disposal.
The process of adding odor to gas
 Gas odorizer
Gas odorizer
Before adding mixtures of mercaptans to the gas pipeline, their quality, concentration, composition and compliance with GOST requirements are checked. After that, the tank is connected to the installation and the additives are pumped into its tank. Then the program is exposed, if the equipment is automatic. In manual mode, the parameters are set on the dispenser in accordance with the characteristics of the mixture and the volume of gas being pumped.
In the future, the flow is switched between installations. Refueled, it begins to supply odorants to the highway. The empty device is stopped, it is serviced, checked, refueled and prepared for further operation.
The operator does not need to check whether the gas has an odor; for this, there are control sensors that determine the concentration of mercaptans in it.